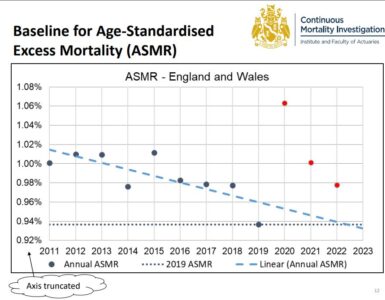
Several countries have chosen to pause administering the AstraZeneca vaccine following reports of blood clots.
According to AstraZeneca, there have been 15 instances of deep vein thrombosis and 22 events of pulmonary embolism reported among more than 17 million people vaccinated in the European Union and UK.
However, it’s worth noting that the annual incidence of venous thromboembolism is approximately 2 in 1,000 of the general population, and the annual incidence of diagnosed pulmonary embolism in the UK has been reported as 7–8 per 10,000 people.
The risk varies substantially with age; for people under 40 years the annual incidence of venous thromboembolism is 1 in 10,000, whereas for people over 80 years the incidence rises to 1 in 100.
Therefore, the reported cases of thromboembolic events being reported in relation to the AstraZeneca vaccine are far fewer than might normally be expected in the absence of any vaccinations.
Whilst medical regulators are no-doubt seeking to apply the precautionary principle, these decisions risk increasing vaccine hesitancy within the relevant countries and beyond.